Well-established safety profile
MOVANTIK has been studied in 1497 patients1-3
Adverse reactions in KODIAC-04 and KODIAC-05 that occurred in ≥3% of patients receiving MOVANTIK 12.5 mg or 25 mg and at an incidence greater than placebo1:
(n=446)
(n=441)
(n=444)
Safety data for KODIAC-07† and KODIAC-08‡ were similar to those observed in KODIAC-04 and KODIAC-05.1-3
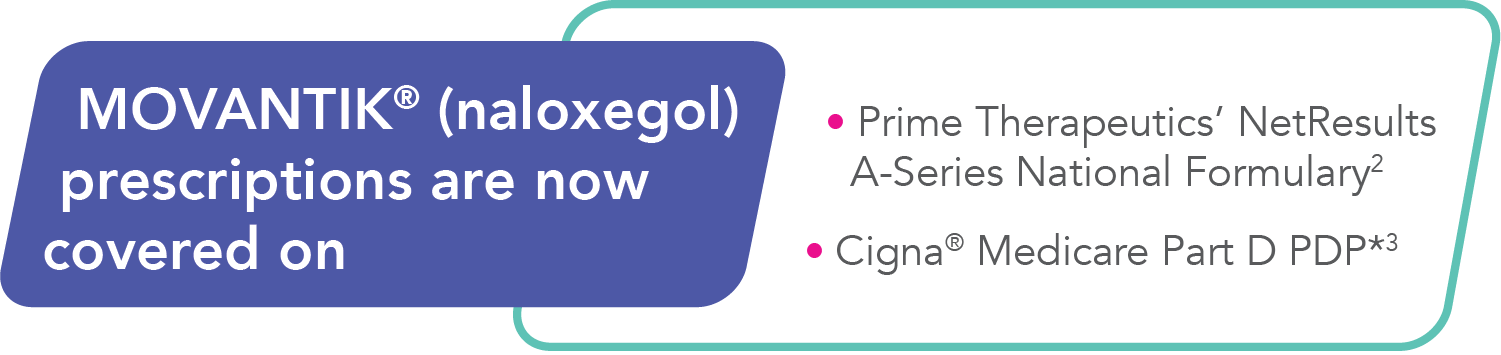
INDICATIONS AND USAGE
MOVANTIK® (naloxegol) is an opioid antagonist indicated for the treatment of opioid-induced constipation (OIC) in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation.
IMPORTANT SAFETY INFORMATION ABOUT MOVANTIK
CONTRAINDICATIONS
- MOVANTIK® is contraindicated in:
- Patients with known or suspected gastrointestinal obstruction and patients at risk of recurrent obstruction, due to the potential for gastrointestinal perforation.
- Patients concomitantly using strong CYP3A4 inhibitors (e.g., clarithromycin, ketoconazole) because these medications can significantly increase exposure to naloxegol, which may precipitate opioid withdrawal symptoms such as hyperhidrosis, chills, diarrhea, abdominal pain, anxiety, irritability, and yawning.
- Patients with a known serious or severe hypersensitivity reaction to MOVANTIK or any of its excipients.
WARNINGS AND PRECAUTIONS
- Opioid withdrawal: Clusters of symptoms consistent with opioid withdrawal, including hyperhidrosis, chills, diarrhea, abdominal pain, anxiety, irritability, and yawning, have occurred in patients treated with MOVANTIK. Additionally, patients receiving methadone as therapy for their pain condition were observed in clinical trials to have a higher frequency of gastrointestinal adverse reactions that may have been related to opioid withdrawal than patients receiving other opioids. Patients having disruptions to the blood-brain barrier may be at increased risk for opioid withdrawal or reduced analgesia. Consider the overall risk benefit in patients with disruptions to the blood-brain barrier. Monitor for symptoms of opioid withdrawal in such patients.
- Severe abdominal pain and/or diarrhea: Reports of severe abdominal pain and/or diarrhea have been reported, some of which resulted in hospitalization. Most of the cases of severe abdominal pain were reported in patients taking the 25 mg dosage. Symptoms generally occurred within a few days of initiation of MOVANTIK. Monitor patients for the development of abdominal pain and/or diarrhea with MOVANTIK and discontinue therapy if severe symptoms occur. Consider restarting MOVANTIK at 12.5 mg once daily, if appropriate.
- Gastrointestinal perforation: Cases of GI perforation have been reported with the use of peripherally acting opioid antagonists, including MOVANTIK. Postmarketing cases of GI perforation, including fatal cases, were reported when MOVANTIK was used in patients at risk of GI perforation (e.g., infiltrative gastrointestinal tract malignancy, recent gastrointestinal tract surgery, diverticular disease including diverticulitis, ischemic colitis, or concomitantly treated with bevacizumab). MOVANTIK is contraindicated in patients with known or suspected gastrointestinal obstruction or in patients at risk of recurrent obstruction. Take into account the overall risk-benefit profile when using MOVANTIK in patients with these conditions or other conditions which might result in impaired integrity of the gastrointestinal tract wall (e.g., Crohn’s disease). Monitor for severe, persistent, or worsening abdominal pain; discontinue MOVANTIK if this symptom develops.
ADVERSE REACTIONS
- The most common adverse reactions in clinical trials (≥3%) are: abdominal pain, diarrhea, nausea, flatulence, vomiting, headache, and hyperhidrosis.
DRUG INTERACTIONS
- Moderate CYP3A4 inhibitors (e.g., diltiazem, erythromycin, verapamil): Increased naloxegol concentrations; avoid concomitant use; if unavoidable, reduce dosage to 12.5 mg once daily and monitor for adverse reactions.
- Strong CYP3A4 inducers (e.g., rifampin, carbamazepine, St. John’s Wort): Decreased concentrations of naloxegol; concomitant use is not recommended.
- Other opioid antagonists: Potential for additive effect and increased risk of opioid withdrawal; avoid concomitant use.
USE IN SPECIFIC POPULATIONS
- Pregnancy and lactation: The use of MOVANTIK during pregnancy may precipitate opioid withdrawal in the pregnant woman and the fetus. Because of the potential for adverse reactions, including opioid withdrawal in breastfed infants, advise women that breastfeeding is not recommended during treatment with MOVANTIK.
- Hepatic impairment: Avoid use of MOVANTIK in patients with severe hepatic impairment, as the dosage in these patients has not been determined. No dosage adjustment is required for patients with mild or moderate hepatic impairment.
- Renal impairment: In patients with creatinine clearance values <60 mL/minute (i.e., moderate, severe, or end-stage renal disease), a lower starting dosage of 12.5 mg once daily is recommended. No dosage adjustment is needed in patients with mild renal impairment.
To report SUSPECTED ADVERSE REACTIONS, contact Valinor at 1-877-592-2337 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Click here for the Medication Guide and full Prescribing Information for MOVANTIK.
This site is intended for US health care professionals only.